How to navigate away from the digital health headwinds
As part of my role as Head of Digital Healthcare UK&I at Bayer, I work with colleagues around the globe to share understandings and developments of digital health technology (DHT). In April 2022, we co-authored a position paper to provide insights into DHT, highlighting why it is important and identifying key barriers to patient access, and providing some proposals on how to address them.
While the purpose was to inform colleagues across our global footprint, the paper contained observations from the UK market that are important in what we see as this current ‘sea of digital change’.
To ensure readers are on the same page, it is always good to confirm we are using the same definitions for digital health, digital medicine, and digital therapeutics. A simple model can be found here.
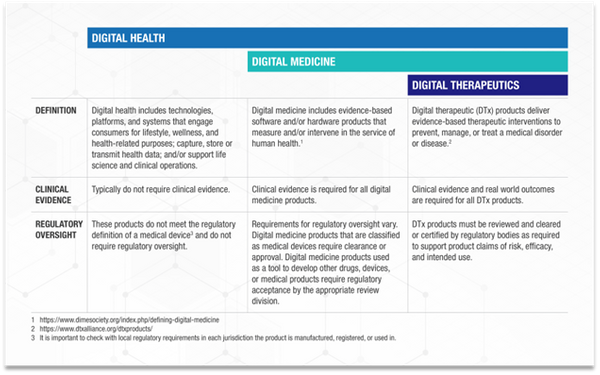
Knowing which classification your DHT offering pertains to is crucial to determine the journey you and your company need to embark on and how to navigate the relevant requirements and challenges.
Sailing through Choppy Waters
Let’s look at some of those key barriers covered in Bayer’s position Paper.
Lack of Fit-For-Purpose Payment Systems
The rapidly growing DHT options require payment systems to evolve to support this growth. The fragmented, ad-hoc and unpredictable approach to reimbursement of DHTs remains a substantial barrier to broad adoption in most healthcare systems. For example, NHS England, has multiple pilot funding streams for DHTs, including the AI in Health and Care Awards, the Digital Health Partnership Award and more recently, the Elective Recovery Technology Fund. While these funding streams are welcome, adequate long-term funding is also critical.
Assessment Methodologies not specific to DHTs
DHTs can provide a broad range of benefits to the healthcare system. They can improve access to care, reduce health inequalities, promote patient autonomy, and reduce inefficiencies. However, they cannot currently be adequately assessed and valued by existing health technology assessment (HTA) methods, primarily developed to assess therapeutic interventions such as medicines and implantable medical devices.
DHTs are updated and on a regular basis, meaning evidence generated from a Randomised Controlled Trial (RCTs) conducted with an older version of the product may already be obsolete by the time it is published. Given the unique ability of DHTs to collect exhaustive data, single-case observational designs (SCODs) may be well-suited to complement and strengthen evidence collected through RCTs. It is important for HTA bodies to consider these aspects and define DHT-specific HTA evaluation methodologies.
Barriers at Physician and Patient Level
A systematic literature review on the role of DHTs in cardiovascular care identified several factors that limit the adoption of DHTs, including:
- Increased physician workload
- Limited digital literacy
- Fear of loss of relationship with health provider
- Lack of awareness or perceived usefulness
- Reluctance to change long-established behaviours
- Lack of clinical leadership and patient ownership
- Physician-patient communication barriers.
Early experience from Germany after the introduction of the digital health applications pathway (DiGA) confirms that adoption is not automatic after reimbursement. Limitations in physician and patient awareness, as well as a reluctance from physicians to prescribe digital therapeutics, make the pathway cumbersome. There is an opportunity for NICE to carve out a new, more agile approach.
Setting the compass to calmer waters
There are several action points industry and policymakers can do to address these issues.
- Provide clear and transparent reimbursement pathways
- Offer adequate and sustainable funding
- Ensure Health Technology Assessment for DHTs is tailored and fit for purpose
- Prioritise Healthcare systems efforts to overcome barriers to patient and staff adoption of DHTs
- Drive innovative partnerships between DHT vendors and healthcare stakeholders
Using a map to chart your journey and stay on course
At Bayer, we welcome the recently published Plan for Digital Health and Social Care, which sets out the government’s commitment to DHT and ambitions; and provides a ‘map’ for industry to use.
The Plan identified several barriers, which have been also recognized by Bayer. These include disparate guidance, with a lack of clarity on how it fits together. A clear need to make it simpler to collaborate with partners in the health and care ecosystem on developing digital solutions that are readily adoptable across the system. Lastly, a long-term view on funding for strategic investment supporting long term transformation.
Companies are not ‘sailing alone’, and we welcome the opportunity to work with organisations including the NICE Office for Digital Health, the NHS Transformation Directorate, and MHRA to name a few to help address the challenges that we’re seeing together.
Acknowledgement: Bayer Digital ACT team

